What Is Clinical Trial Management?
Clinical trial management refers to the structured, organized regulatory approach that managers take in clinical trial projects to produce timely and efficient project outcomes. It includes developing and maintaining specified or general software systems, processes, procedures, training, and protocols.
What Is a Clinical Trial Management System (CTMS)?
A clinical trial management system (CTMS) is a type of project management software specific to clinical research and clinical data management. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether across one or several institutions.
Companies use CTMS for their clinical data management to ensure they build trust with regulatory agencies. Trust is earned as the companies collect, integrate, and validate their clinical trial data with integrity over time. A comprehensive system helps them do so.
In a 2017 paper, “Artificial intelligence based clinical data management systems: A review,” Gazali discusses CTMS and what makes it worthwhile for investigators — namely, that it helps to authenticate data. Accurate study results and a trail of data collection, as collected through a quality CTMS, lend credence to research study data. Clinical trial data management systems enable researchers to adhere to quality standards and provide assurance that they are appropriately collecting, cleaning, and managing the data.
A clinical data management system also offers remote data monitoring. The sponsor, or principal investigator, may want to monitor the trial from a distance, especially if the organization has many sites. Since the FDA mandates monitoring in clinical trials, and many studies generally consider it a large cost, remote monitoring offers a lower-priced option in which sponsors can identify issues and outliers and mitigate them quickly.
Many data management systems are also incorporating artificial intelligence (AI). AI-based clinical data management systems support process automation, data insights analysis, and critical decision making. All of this can happen as your staff inputs the research data. According to a review of clinical data management systems, researchers note that automating all dimensions of clinical data management in trials can take them from mere electronic data capture to something that helps with findings in clinical trials.
The most helpful strategies for implementing clinical data management systems balance risk reduction and lead time. All trial managers want to have their software deployed rapidly. However, it is best to set up the databases thoroughly before the trial. When staff must make software changes during the trial, it can be costly and have implications on the trial data’s validity.
Other strategies that help organizations implement a new system include making sure that, prior to deployment, the intended users give input. These users include entities such as the contract research organization (CRO), the sponsor, staff at the investigator site, and any onsite technical support. Staff should respond well to the graphical user interface (GUI). Additionally, depending on software support, the staff can gradually expand the modules to include more functionality, perform module-based programming, and duplicate the hardware. These actions give the staff the most functionality and the software the best chance at success.
How to Compare Clinical Data Management Systems
When deciding which clinical data management system to use, compare the program’s available features and those that your clinical sites need. Additionally, you can compare clinical data management systems by reviewing the installation platforms, pricing, technical support, and number of allowed users.
For programs that collect data on paper and send it to data entry staff, the data entry portal should be simple and allow for double entry and regular oversight.
In general, here are the main features to compare in a clinical data management system:
- 21 CFR Part 11 Compliance: Electronic systems must provide assurance of authentic records.
- Document Management: All documents should be in a centralized location, linked to their respective records.
- Electronic Data Capture (EDC): Direct clinical trial data collection, as opposed to paper forms.
- Enrollment Management: Research studies can use this data (from interactive web or voice response systems) to enroll, randomize, and manage patients.
- HIPAA compliance: Ensure compliance with the Health Insurance Portability and Accountability Act to protect patients’ information.
- Installation: Identify whether you want a cloud-based or on-premises solution and if you need mobile deployment (iOS or Android).
- Investigator and Site Profiling: Use this function to rapidly identify the feasibility of possible investigators and sites.
- Monitoring: The system should offer a calendar, scheduling capabilities, adverse and severe adverse event tracking, trip reporting, site communication, and triggers.
- Number of Users: How many users can the software can handle? Is there a minimum number of required users? Does the software provide levels of accessibility and price based on the number of users?
- Patient Database: Separate from recruitment and enrollment, the patient database is a record of previous contacts that you can potentially draw from for future trials.
- Payment Portal: Pay out stipends, contracts, and other finances related to the research project.
- Pricing: Check whether the software company offers free trials, free or premium options, monthly subscriptions, annual subscriptions, or a one-time license.
- Recruiting Management: This function helps streamline recruitment by targeting potential trial patients with web recruitment and prescreening.
- Scheduling: Use this feature to keep track of visits and events.
- Study Planning and Workflows: This function enables you to track all required study pieces from the beginning and optimize each piece with workflows.
- Support: Check if the software company offers 24-hour issue support and training on the software.
What Is Clinical Data Management?
Clinical data management (CDM) is the part of clinical trial management that deals specifically with information that comes out of the trials. In order to yield ethical, repeatable results, researchers must document their patients’ medical status — including everything relative to that status — and the trial’s interventions.
Clinical data management evolved from drug companies’ need for an honest path from their research to their findings; in short, their data had to be reproducible. CDM helps evolve a standards-based approach, and many regulators are continually imposing their requirements on it. For instance, paper is no longer favored as a collection method; most clinical trials prefer software systems that improve the timeliness and quality of data.
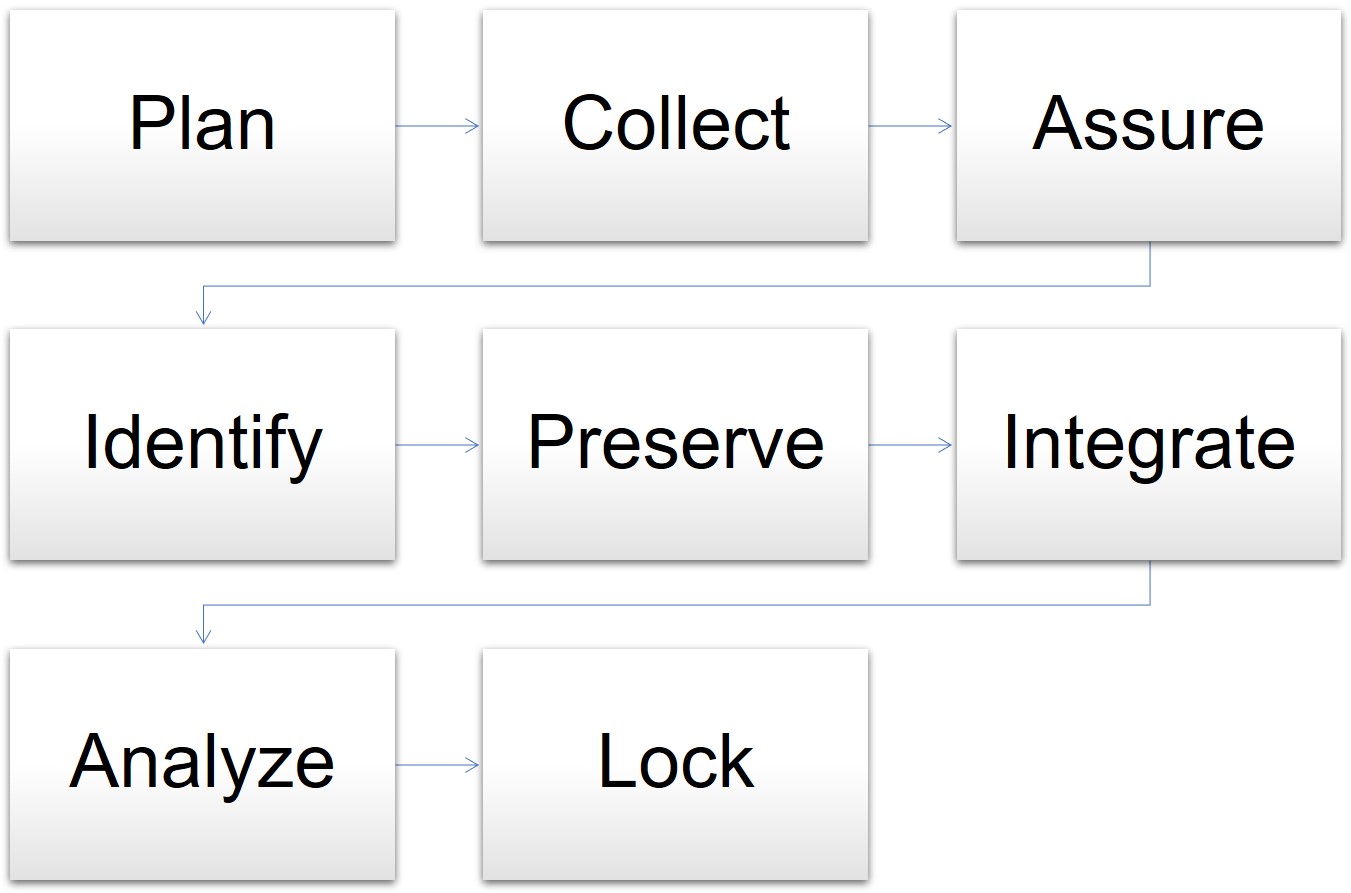
In one model for data management, the cycle begins when the clinical trial is in the planning stages and goes through the final analysis and lockdown of the data. The stages for data management are as follows:
- Plan: The data manager prepares the database, forms, and overall plan.
- Collect: Staff gathers data in the course of the trial.
- Assure: The data manager determines if the data plan and tools meet the requirements.
- Identify: Staff and the data manager identify any issues or risks.
- Preserve: The data manager preserves the data already collected and mitigates risks.
- Integrate: The data manager oversees different datasets and information mapped together for consistency.
- Analyze: The statisticians analyze the mapped data trends and outcomes.
- Lock: The data manager locks the database for integrity.
Model for Data Management in Clinical Trials
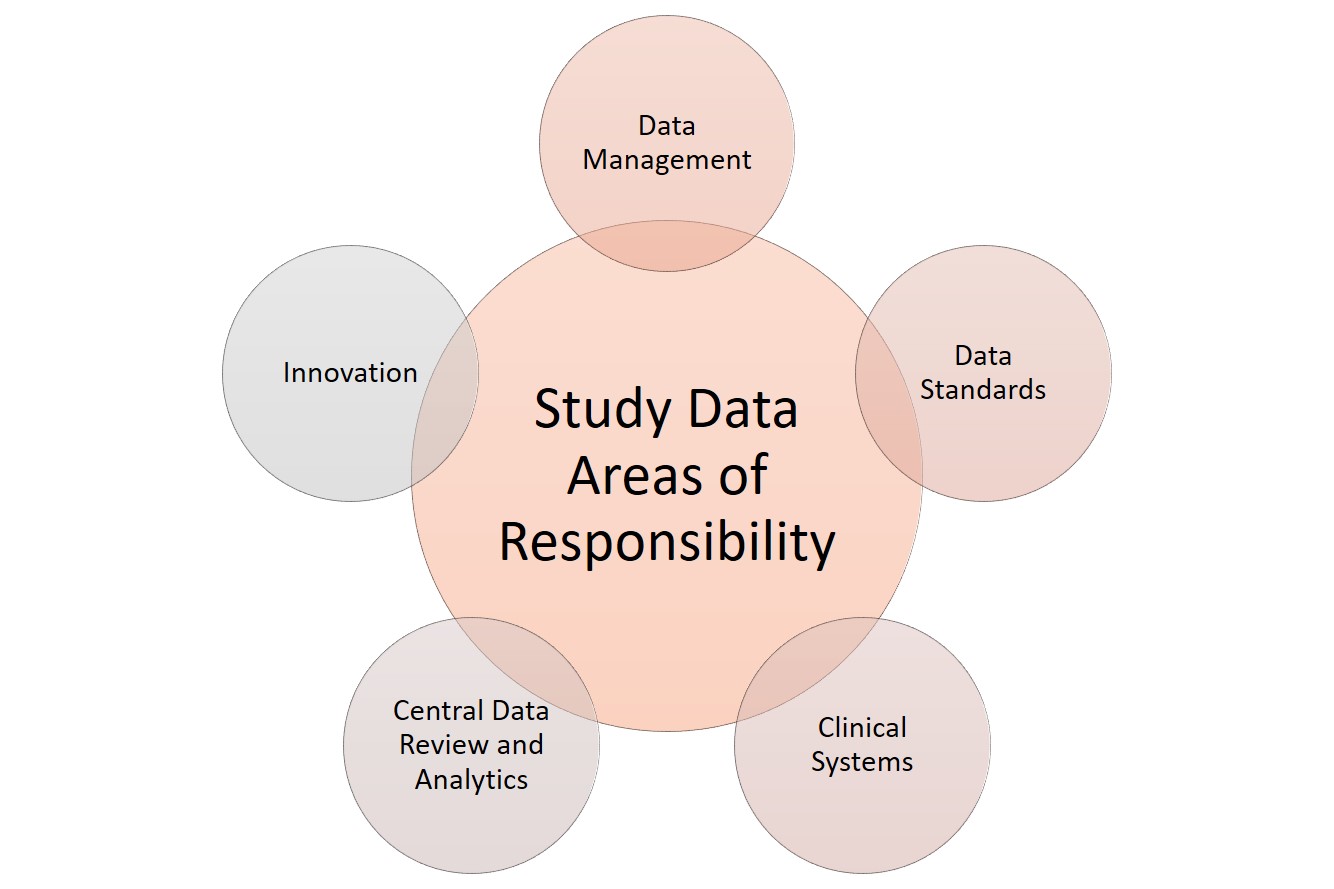
When it comes to data, clinical research has several areas of responsibility. Sponsors can split these functions among several staff or, in smaller studies, assign them to the main data manager. These functions include the following:
-
Clinical systems: Any software or technology used.
-
Data management: Data acquisition, coding, and standardization.
-
Data review and analytics: Quality management, auditing, and statistical analysis of the collected data.
-
Data standards: Checking against regulatory requirements.
-
Innovation: Using tools and theory that coordinate with the developing field. For more innovative templates to use in clinical trials, see “Clinical Trial Templates to Start Your Clinical Research.”
Clinical Research Data Areas of Responsibility
Clinical data management is one of the most critical functions in overall clinical trial management. Staff collects data from many different sources in a clinical trial — some will necessarily be from paper forms filled out by the patient, their representative, or a staff member on their behalf. However, instead of paper, some clinics may use devices such as tablets or iPads to fill out this direct-entry data electronically.
Clinical data management also includes top-line data, such as the demographic data summary, the primary endpoint data, and the safety data. Together, this constitutes the executive summary for clinical trials. Companies often issue this data as a part of press releases. Additional clinical trial data management activities include the following:
- Case report form (CRF) design, annotation, and tracking
- Central lab data
- Data abstraction and extraction
- Data archiving
- Data collection
- Data entry and validation
- Data extraction
- Data queries and analysis
- Data storage and privacy
- Data transmission
- Database design, build, and testing
- Database locking
- Discrepancy management
- Medical data coding and processing
- Patient recorded data
- Severe adverse event (SAE) reconciliation
- Study metrics and tracking
- Quality control and assurance
- User acceptance testing
- Validation checklist
Since there are many different types of data coming from many different sources, some data managers have become experts in hybrid data management — the synchronization required to not only make disparate data relate to each other, but also to adequately manage each type of data. For example, one study could generate data on paper from both the trial site and from a contract research organization, electronic data from the site, and clinical data measurements from a laboratory.
The Roles and Responsibilities in Clinical Data Management
Clinical data management software assigns database access limitations based on the assigned roles and responsibilities of the users. This coding ensures there is an audit trail and the users can only access their respective required functionalities, without the ability to make other changes.
All staff members, whether a manager, programmer, administrator, medical coder, data coordinator, quality control staff, or data entry person, have differing levels of access to the software system, as delineated in the protocol. The principle investigator can use the CDMS to restrict these access levels.
What Is Clinical Trial Data Management (CDM)?
Clinical trial data management (CDM) is the process of a program or study collecting, cleaning, and managing subject and study data in a way that complies with internal protocols and regulatory requirements. It is simultaneously the initial phase in a clinical trial, a field of study, and an aspirational model.
With properly collected data in clinical trials, the study can progress and result in reliable, high-quality, statistically appropriate conclusions. Proper data collection also decreases the time from drug development to marketing. Further, proper data collection involves a multidisciplinary team, such as the research nurses, clinical data managers, investigators, support personnel, biostatisticians, and database programmers. Finally, CDM enables high-quality, understandable research, which can be capitalized on in its field and across many disciplines, according to the National Institutes of Health (NIH).
In clinical trials, data managers perform setup during the trial development phase. Data comes from the primary sources, such as site medical records, laboratory results, and patient diaries. If the project uses paper-based CRFs, staff members must transcribe them, then enter this source data into a clinical trial database. They enter paper-based forms twice, known as double data entry, and compare them, per best practice. This process significantly decreases the error rate from data entry mistakes. Electronic CRFs (eCRFs) enable staff to enter source data directly into the database.
As with any project, the financial and human resources in clinical trials are finite. Coming up with and sticking to a solid data management plan is crucial — it should include structure for the research personnel, resources, and storage. A clinical trial is a huge investment of time, people, and money. It warrants expert-level management from its inception.
Clinical Data Management Plans
Clinical data management plans (DMPs) outline all the data management work needed in a clinical research project. This includes the timeline, any milestones, and all deliverables, as well as strategies for how the data manager will deal with disparate data sets.
Regulators do not require a DMP, but they expect and audit them in clinical research. Thus, the DMPs should be comprehensive and all stakeholders should agree on them. They should also be living documents that staff regularly updates as the study evolves and the various study pieces develop.
For example, during one study, the study manager might change the company used for laboratory work. This affects the DMP in two ways: First, staff needs to develop the data sharing agreement with the new company, and second, they need to integrate the data from both laboratories into one dataset at the end of the trial. The DMP should describe both.
When creating DMPs, you should also bear in mind any industry data standards, so the research can also be valuable outside of the discrete study. The Clinical Data Acquisitions Standards Harmonization (CDASH) recommends 16 standards for data collection fields for consistency in data across different studies.
The final piece of standardization in DMPs is the use of a template, which provides staff with a solid place to start developing a DMP specific to their study. Sponsors may have a standard template they use across their projects to help reduce the complexity inherent in clinical trials.
Data Management Plan Template for Clinical Trials
This data management plan template provides the required contents of a standard clinical trial data management plan, with space and instructions to input elements such as the data validation process, the verification of database setup and implementation processes, and the data archival process.
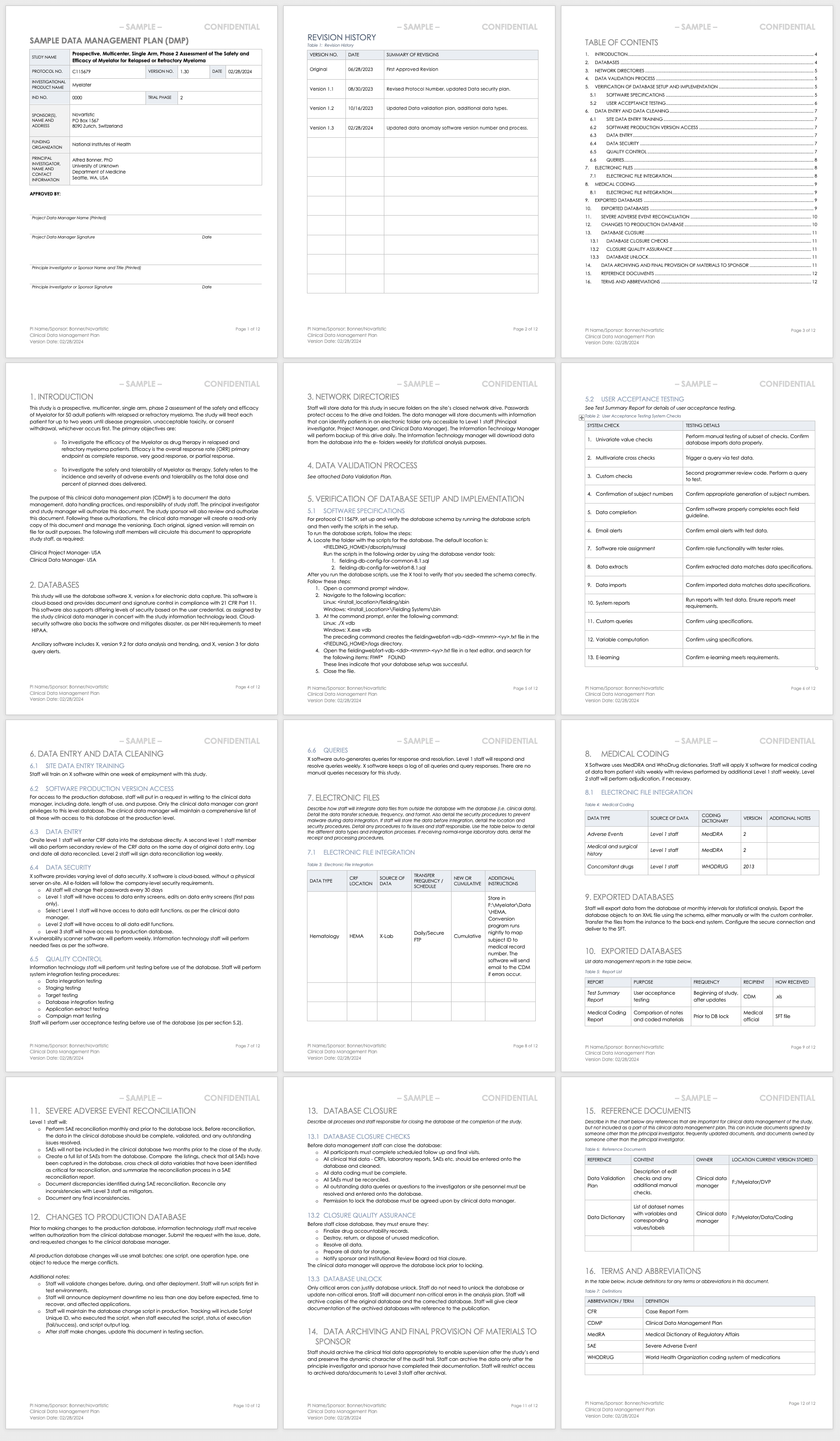
Sample Data Management Plan for Clinical Trials
This sample data management plan shows a fictitious prospective, multicenter, single-arm study and its data management process needs. In two years of study, the data manager should regularly update this plan to demonstrate the study’s evolving needs, and document each change and update. Examples of sections include the databases used, how data will be entered and cleaned, and how staff will integrate different data sets collected in the study.
Clinical Trial Data Validation Plan
Data validation involves resolving database queries and inconsistencies by checking the data for accuracy, quality, and completeness. A data validation plan in clinical trials has all the variable calculations and checks that data managers use to identify any discrepancies in the dataset.
When the data is final, the database administrator locks it to ensure no further changes are made, as they could interrupt the integrity of the data. During reporting and analysis, experts may copy the data and reformat it into tables, lists, and graphs. Once the analysts complete their work, they report the results. When they have significant findings, they may create additional tables, lists, and graphs to present as part of the results. They then integrate these results into higher-level findings documentation. Examples of this type of documentation include investigator’s brochures or clinical case study reports (CSRs). Finally, the data manager archives the database.
The above steps are important because they preserve the integrity of the data in the database. However, managers do not need to perform them in a strict order. Some studies may need more frequent data validation, due to the high volume of data they produce, while other studies may produce intermediate analysis and reporting as part of their predetermined requirements. Finally, due to the complexity of some studies, the data manager or analyst may need to query, which means running a data request in a database and determining cursory results so that they may adjust the protocol.
Use this template to develop your own data validation plan. This Word template includes space and instructions for you to develop a data validation plan that you can include in your data management plan or use as a stand-alone document. Examples of sections include selecting and classifying the computer systems, validation protocol, and validation reporting.
Data Management Workflow
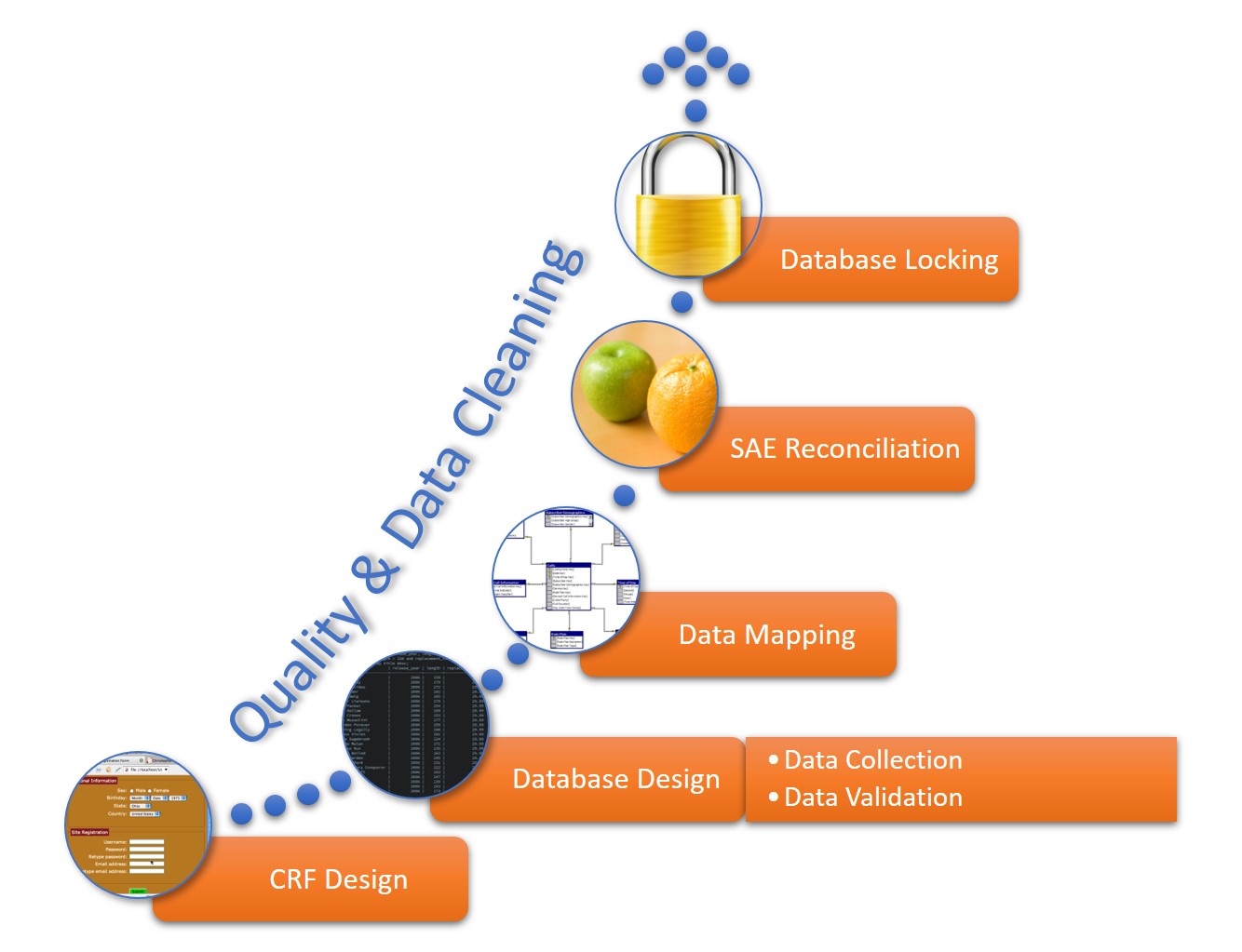
A data management workflow is the process clinical research uses to deal with their data, from the data collection design to the electronic archival and findings presentation. This includes getting through the entry process, any batch validation, discrepancy management, coding, reconciliations, and quality control plans.
This workflow starts when researchers generate a CRF, whether manually or electronically, and continues through the final lock on the database. The data manager should perform quality checks and data cleaning throughout the workflow. The workflow steps for a data manager are as follows:
- CRF Design: This initial design step forms the basis of initial data collection.
- Database Design: The database should include space for all data collected in the study.
- Data Mapping: This step integrates data from different forms or formats so researchers can consistently report it.
- SAE Reconciliation: Data managers should regularly review and correct severe adverse events and potential events.
- Database Locking: Once a study is complete, the database manager should lock the database so that no one can change the data.
Clinical Trial Data Audits
A clinical trial data audit is a review of the information collected in order to ensure the quality, accuracy, and appropriateness for the stated research requirements, per the study protocol. Regulatory authorities, sponsors, and internal study staff can conduct two varieties of audit: overall and database-specific.
Regulators use database audits to ensure that no one has tampered with the data. In general, there must be an audit trail to know which user made changes to what and when in the database. For example, the auditors will look at record creation, modification, and deletion, noting the usernames, dates, and times. FDA 21 CFR Part 11 includes this as a part of fraud detection, and requires that there is a complete history of the recordkeeping system and clinical trial data transparency.
The data manager develops templates for auditing the study during the study development phase and performs their own internal audits as a part of its quality management.
This free clinical trial data management audit checklist template will help you develop your own checklist. This Excel template lets you show the status of your audit in an easy color-coded display, the category and tasks to review, and what criteria you require. It brings all your audit requirements and results together.
Quality Management in Clinical Trials
Data quality management (DQM) refers to the practices that ensure clinical information is of high value. In a clinical trial, DQM starts when staff first acquires the information and continues until the findings are distributed. DQM is critical in providing accurate outcomes.
The factors that influence the quality of clinical data include how well the study investigators develop and implement each of the following data pieces:
- Case Report Forms (CRF): Design the CRF in parallel with the protocol so that the data collected by staff is complete, accurate, and consistent.
- Data Conventions: Data conventions include dates, times, and acronyms. Data managers should set these conventions during study development, especially if there are multiple study locations and investigators.
- Guidelines for Monitoring: The overall data quality is contingent on the quality of the monitoring guidelines established.
- Missing Data: Missing data are those values not available that could change the analysis findings. During study development, investigators and analysts should determine how they will handle missing data.
- Verification of Source Data: Staff must verify that the source data is complete and accurate during data validation.
Regulations, Guidelines, and Standards in Clinical Data Management
Different regulations, guidelines, and standards govern clinical data management industry. The Clinical Data Interchange Standards Consortium (CDISC) is a global organization that holds clinical studies accountable to clinical trial data standards, international regulations, institutional and sponsor standard operating procedures (SOPs), and state laws.
There are standard operating procedures and best practices in clinical trial data management that are widespread. CDISC has two standards, the Study Data Tabulation Model Implementation Guide for Human Clinical Trials (SDTMIG), mandated by the U.S. Food and Drug Administration (FDA), and the Clinical Data Acquisition Standards Harmonization (CDASH). Also, in the industry, the Society for Clinical Data Management (SCDM) releases the Good Clinical Data Management Practices (GCDMP) guidelines and administers the International Association for Continuing Education and Training (IACET) credential for certified clinical data managers. The National Accreditations Board of Hospitals Health (NABH) provides additional guidance, such as pharmaceutical study auditing checklists. Finally, Good Clinical Practices (GCP) guidelines discuss ethical and quality standards in clinical research.
A trial conducted under the appropriate standards ensures that staff has followed the protocol and treated the patients according to that protocol. Ultimately, this shows the integrity and reproducibility of the study and acceptance in the industry.
Case Report Forms in Data Management
In data management, CRFs are the main tool researchers use to collect information from their participants. Researchers design CRFs based on the study protocol; in them, they document all patient information per the protocol for the duration of the study’s requirements.
CRFs should comply with all regulatory requirements and enable efficient analysis to decrease the need for data mapping during any data exchange. When longer than one page, the CRF is known as a CRF book, and each visit adds to the book. The main parts of a CRF are the header, the efficacy-related modules, and the safety-related modules:
- CRF Header: This portion includes the patient identification information and study information, such as the study number, site number, and subject identification number.
- Efficacy-Related Modules: This portion includes the baseline measurements, any diagnostics, the main efficacy endpoints of the trial, and any additional efficacy testing.
- Safety-Related Modules: The portion contains the patient’s demographic information, any adverse events, medical history, physical history, medications, confirmation of eligibility, and any reasons for release from the study.
What Is the Role of a Clinical Data Manager?
In a clinical trial, the data manager is the person who ensures that the research staff collects, manages, and prepares the resulting information accurately, comprehensively, and securely. This is a key role in clinical research, as the person is involved in the study setup, conduct, closeout, and some analysis and reporting.
Melissa Peda, Clinical Data Manager at Fred Hutch Cancer Research Center, says, “Being a clinical data manager, you have to be very detail-oriented. We write up very specific instructions for staff. For example, the specifications to a program’s database include one document that could easily have 1,000 rows in Excel, and it needs to be perfect for queries to fire in real time. Code mistakes can put your project behind, so they must do their review with a close eye. You must also be logical and think through the project setup. A good clinical data manager must be detailed, so the programmers and other staff can do their thing.”
Krishnankutty, et al., developed an overview of best practices for data management in clinical research. In their article, published in the Indian Journal of Pharmacology, they say that the need for strong clinical data management has sprung up from the pharmaceuticals industry wanting to fast-track drug development by having high-quality data, regardless of the type of data. Clinical data managers can expect to work with many different types of clinical data; the most common types include the following:
- Administrative data
- Case report forms (CRFs)
- Claim data
- Electronic health records
- Laboratory data
- Patient diaries
- Patient or disease registries
- Safety data
The clinical data managers often must oversee the analysis of the data as well. Data analysis conducted in clinical trial data management is very delicate: It requires a solid dataset and an analyst who can explain the findings. Regulatory agencies, along with other companies and professionals, check the findings and analysis, so they need to be accurate and understandable.
Education and Credentials of a Clinical Data Manager
Professionals in clinical data management receive data management in clinical trials training, and often have the Certified Clinical Data Manager (CCDM) credential. Their studies can have optimized outcomes since they are executed by a competent CDM team with validated skill sets and continued professional development.
To become certified, the applicant must have the appropriate education and experience, including the following:
- A bachelor’s degree and two or more years of full-time data management experience.
- An associate’s degree and three or more years of full-time data management experience.
- Four years of full-time data management experience.
- Part-time data management experience that adds up to the requirements above.
Raleigh Edelstein, a clinical data manager and EDC programmer, discusses the credentialing in this field. “Anyone can excel in this profession,” she says. “A CRA — a clinical research associate, also called a clinical monitor or a trial monitor — may need this credential more, as their profession is more competitive, and their experience is more necessary in trials. But if the credential makes you more confident, then I say go for it. Your experience and confidence matter.”
There are several degrees with an emphasis on clinical research that can also teach the necessary technical skills. In addition to many online options, these include the following, or a combination of the following:
- Associate of Science in biology, mathematics, or pharmacy.
- Bachelor of Science in one of the sciences.
- Post-Master's certificate in clinical data management, or a certificate related to medical device and drug development.
- Master of Science in clinical research, biotechnology, bioinformatics.
- Doctor of Nursing Practice.
- Doctor of Philosophy in any clinical research area.
These degree programs include concepts that help data managers understand what clinical studies need. They especially focus on survey design and data collection, but also include the following:
- Biostatistics
- Clinical research management and safety surveillance
- Compliance, ethics, and law
- New product business and strategic planning
- New product research and development
These degree programs offer coursework that improves the relevant clinical research skills. Many of the courses are introductory to clinical research, trials, and pharmacology, and others include the following:
- Auditing
- Business processes
- Clinical outsourcing
- Clinical research biostatistics
- Clinical trial design
- Compliance and monitoring
- Data collection strategies
- Data management
- Electronic data capture
- Epidemiology
- Ethics in research
- Federal regulatory issues
- Health policy and economics
- Human research protection
- Medical devices and product regulation
- Patient recruitment and informed consent
- Pharmaceutical law
- Review boards
- Worldwide regulations for submission
Clinical data managers can get involved with several professional organizations worldwide, including the following:
- The Association for Clinical Data Management (ACDM): This global professional organization supports the industry by providing additional resources and promoting best practices.
- The Association Française de Data Management Biomédicale (DMB): This French data management organization shares information and practices among professionals.
- International Network of Clinical Data Management Associations (INCDMA): Based in the United Kingdom, this professional network exchanges information and discusses relevant professional issues.
- The Society for Clinical Data Management (SCDM): This global organization awards CCDM credential to professionals, provides additional education, and facilitates conferences in clinical data management.
FAQs about Clinical Trial Managers
The field of clinical management is quickly expanding in many forms to support the need for new research. Below are some frequently asked questions.
How do I become a clinical trial manager?
To become a clinical trial manager, you must obtain the appropriate education, experience, and credentialing, as detailed above.
What is better: a Master’s in Health Administration or a Master’s in Health Sciences?
To work as a clinical data manager, either degree program is appropriate. Your choice depends on your interest.
What can you do with a degree in biotechnology or bioenterprise?
Biotechnology is involved in the technology that aids in biological research, and bioenterprise takes the products of biotechnology and markets and sells them.
What is a clinical application analyst?
A clinical application analyst is a professional who helps clinics evaluate software systems and vendors.
What is a clinical data analyst?
A clinical data analyst is a professional who analyzes data from clinical trials, and develops and maintains databases.
Contract Research Organizations for Data Management Services
Contract research organizations (CROs) are companies that provide outsourced research services to industries such as pharmaceutical, biotechnology, and research development. Designed to keep costs low, studies can hire them to perform everything from overall project management and data management to technical jobs.
Studies can hire CROs that specialize as clinical trial data management companies so they don’t have to worry about having all the necessary skills in-house. According to Melissa Peda, “A consultant may have the expertise that someone already working in the organization may not have, so they make sense to bring in.” Further, a contractor outside of the business can bring a lack of bias to the project.
According to Raleigh Edelstein, “A third-party person in charge of data management may be necessary because you don’t have to worry about the lack of company loyalty that the data may need.”
CROs can offer skills such as the following:
- Annotation and review
- Coding and validation
- Database export, transfer, and locking
- Database integration
- Database setup and validation
- Double data entry and third-party review of discrepancies
- Form design
- Management
- Planning, such as project management and data management plans
- Quality assessments and auditing
- Software implementation and training
- SAE reconciliation
Related Topics in Clinical Data Management
The following are related topics to clinical data management:
- Application Analyst: This position deals with the software side of clinical trials. Examples of their work include choosing software, designing databases, and writing queries.
- Clinical Data Analyst: A professional who examines and verifies that clinical study data is appropriate and means what it is supposed to mean.
- Clinical Research Academic Programs: Entry-level professional positions in clinical trials often require a minimum of a bachelor’s degree.
- Clinical Research Associate: This clinical trial staff member designs and performs clinical studies.
- Laboratory Informatics: The field of data and computational systems specialized for laboratory work.
- Laboratory Information Management System (LIMS): LIMS enables collection and analysis of data from laboratory work. LIMS is specialized to work in different environments, such as manufacturing and pharmaceuticals.
- Scientific Management: This management theory studies workflows, applying science to process engineering and management.
Improve Clinical Trial Data Management with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Any articles, templates, or information provided by Smartsheet on the website are for reference only. While we strive to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability, or availability with respect to the website or the information, articles, templates, or related graphics contained on the website. Any reliance you place on such information is therefore strictly at your own risk.
These templates are provided as samples only. These templates are in no way meant as legal or compliance advice. Users of these templates must determine what information is necessary and needed to accomplish their objectives.